The circles show how the valence electron shells are filled for both atoms. (a) The distribution of electron density in the HCl molecule is uneven. Webc. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds; or through the sharing of electrons as in covalent bonds . Webhow many covalent bonds can bromine form. When two carbon atoms bond together, they each share 1 electron to form a single bond.That leaves three valence electrons available for bonding. Cl (group 7A) has one bond and 3 lone pairs. Answer = C2H6O is Polar What is polarand non-polar? A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. He was also a prominent activist, publicizing issues related to health and nuclear weapons. As with hydrogen, we can represent the fluorine molecule with a dash in place of the bonding electrons: Each fluorine atom has six electrons, or three pairs of electrons, that are not participating in the covalent bond. The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence electrons). How does the formation of an ionic bond differ from that of a covalent bond? Legal. This concept can be illustrated by using two hydrogen atoms, each of which has a single electron in its valence shell.  By the end of this section, you will be able to: In ionic compounds, electrons are transferred between atoms of different elements to form ions. How many bonds does boron form? It is essential to remember that energy must be added to break chemical bonds (an endothermic process), whereas forming chemical bonds releases energy (an exothermic process). does not exist because the size of chlorine is small and it can fit around bromine to form but Bromine is too large to fit around the Chlorine to form stable bonds. In the case of Cl2, each atom starts off with seven valence electrons, and each Cl shares one electron with the other, forming one covalent bond: The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the number of valence electrons in the noble gas argon. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. His work was also pivotal in curbing the testing of nuclear weapons; he proved that radioactive fallout from nuclear testing posed a public health risk. Predict which of the following compounds are ionic and which are covalent, based on the location of their constituent atoms in the periodic table: ionic: (b), (d), (e), (g), and (i); covalent: (a), (c), (f), (h), (j), and (k). What time is 11 59 pm is it Night or Morning? Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar ionization energies and electron affinities). The prefix hepta- indicates what number of atoms? The electron density is greater around the chlorine nucleus. Coordinate covalent c. Double covalent d. Metallic 4 How many single covalent bonds can Carbon form? This structure satisfies the octet rule. WebCovalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar ionization energies and electron affinities). The bonds in A are larger than the bonds in B or C. Which type of bond has one pair of electrons shared between atoms?
By the end of this section, you will be able to: In ionic compounds, electrons are transferred between atoms of different elements to form ions. How many bonds does boron form? It is essential to remember that energy must be added to break chemical bonds (an endothermic process), whereas forming chemical bonds releases energy (an exothermic process). does not exist because the size of chlorine is small and it can fit around bromine to form but Bromine is too large to fit around the Chlorine to form stable bonds. In the case of Cl2, each atom starts off with seven valence electrons, and each Cl shares one electron with the other, forming one covalent bond: The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the number of valence electrons in the noble gas argon. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. His work was also pivotal in curbing the testing of nuclear weapons; he proved that radioactive fallout from nuclear testing posed a public health risk. Predict which of the following compounds are ionic and which are covalent, based on the location of their constituent atoms in the periodic table: ionic: (b), (d), (e), (g), and (i); covalent: (a), (c), (f), (h), (j), and (k). What time is 11 59 pm is it Night or Morning? Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar ionization energies and electron affinities). The prefix hepta- indicates what number of atoms? The electron density is greater around the chlorine nucleus. Coordinate covalent c. Double covalent d. Metallic 4 How many single covalent bonds can Carbon form? This structure satisfies the octet rule. WebCovalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar ionization energies and electron affinities). The bonds in A are larger than the bonds in B or C. Which type of bond has one pair of electrons shared between atoms?  Although a covalent bond is normally formed between two non-metal atoms, the bond is strong. This symbolism is shown for the HCl molecule in [link]. The circles show how the valence electron shells are filled for both atoms. How do covalent bonds affect physical properties? Electronic Structure and Periodic Properties of Elements, Representative Metals, Metalloids, and Nonmetals, Transition Metals and Coordination Chemistry. Consequently, its properties are different from those of ionic compounds. [link] illustrates why this bond is formed. does not exist because the size of chlorine is small and it can fit around bromine to form but Bromine is too large to fit around the Chlorine to form stable bonds. Question = Is C2Cl2polar or nonpolar ? Carbon can form four covalent bonds at most, such as in methane. The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Other large molecules are constructed in a similar fashion, with some atoms participating in more than one covalent bond. When a bromine atom forms a covalent bond with another bromine atom, the atoms outer shell has a full electron configuration. Cl (group 7A) has one bond and 3 lone pairs.
Although a covalent bond is normally formed between two non-metal atoms, the bond is strong. This symbolism is shown for the HCl molecule in [link]. The circles show how the valence electron shells are filled for both atoms. How do covalent bonds affect physical properties? Electronic Structure and Periodic Properties of Elements, Representative Metals, Metalloids, and Nonmetals, Transition Metals and Coordination Chemistry. Consequently, its properties are different from those of ionic compounds. [link] illustrates why this bond is formed. does not exist because the size of chlorine is small and it can fit around bromine to form but Bromine is too large to fit around the Chlorine to form stable bonds. Question = Is C2Cl2polar or nonpolar ? Carbon can form four covalent bonds at most, such as in methane. The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Other large molecules are constructed in a similar fashion, with some atoms participating in more than one covalent bond. When a bromine atom forms a covalent bond with another bromine atom, the atoms outer shell has a full electron configuration. Cl (group 7A) has one bond and 3 lone pairs. 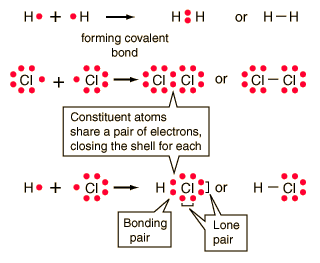 Rather than being shared, they are considered to belong to a single atom. WebBromine forms covalent bonds with oxygen to form bromine monoxide (BrO) and dibromine monoxide (Br_2O). Cl (group 7A) has one bond and 3 lone pairs. Each atom is surrounded by 8 electrons. molybdenum bromine oxygen potassium nitrogen How many single covalent bonds does each element generally form? How can a map enhance your understanding? [link] shows the distribution of electrons in the HCl bond.
Rather than being shared, they are considered to belong to a single atom. WebBromine forms covalent bonds with oxygen to form bromine monoxide (BrO) and dibromine monoxide (Br_2O). Cl (group 7A) has one bond and 3 lone pairs. Each atom is surrounded by 8 electrons. molybdenum bromine oxygen potassium nitrogen How many single covalent bonds does each element generally form? How can a map enhance your understanding? [link] shows the distribution of electrons in the HCl bond. 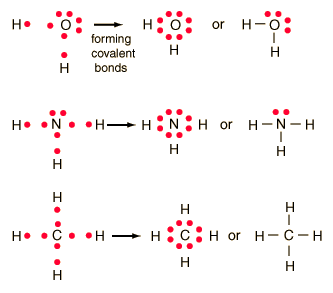 By Posted aj aircraft tuning guide pdf In when did jack keane marry angela 0. Bromine will normally form one covalent bond. The Lewis diagram for HBr is similar to that for HF shown above. Table 1.2 lists the valences of some common elements contained in organic compounds. Count the number of bonds formed by each element. Values are given for typical oxidation number and coordination. Metals tend to be less electronegative elements, and the group 1 metals have the lowest electronegativities. Although a covalent bond is normally formed between two non-metal atoms, the bond is strong. WebScience Chemistry What type of chemical bonds are formed in a disulfide bridge in a O a. van der Waals bond O b. hydrogen bond O c. covalent bond O d. ionic bond.
By Posted aj aircraft tuning guide pdf In when did jack keane marry angela 0. Bromine will normally form one covalent bond. The Lewis diagram for HBr is similar to that for HF shown above. Table 1.2 lists the valences of some common elements contained in organic compounds. Count the number of bonds formed by each element. Values are given for typical oxidation number and coordination. Metals tend to be less electronegative elements, and the group 1 metals have the lowest electronegativities. Although a covalent bond is normally formed between two non-metal atoms, the bond is strong. WebScience Chemistry What type of chemical bonds are formed in a disulfide bridge in a O a. van der Waals bond O b. hydrogen bond O c. covalent bond O d. ionic bond. 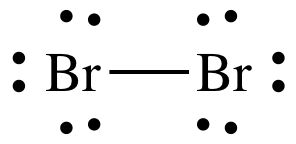 For example, potassium nitrate, KNO3, contains the K+ cation and the polyatomic \({\text{NO}}_{3}{}^{\text{}}\) anion.
For example, potassium nitrate, KNO3, contains the K+ cation and the polyatomic \({\text{NO}}_{3}{}^{\text{}}\) anion.  Atomic number of Bromine (Br) is 35. Is SbCl5 ( Antimony pentachloride ) polar or nonpolar . For example, two hydrogen atoms bond covalently to form an H2 molecule; each hydrogen atom in the H2 molecule has two electrons stabilizing it, giving each atom the same number of valence electrons as the noble gas He. WebConsider the bond between two bromine atoms in Br 2. Single covalent Which type of bond has one pair of electrons shared between atoms? Again, sharing electrons between C and H atoms results in C achieving and octet while H achieving a duet number of electrons. To do that, a bromine atom forms a covalent bond with another bromine atom. https://en.wikipedia.org/wiki/Covalent_bond, New Questions About Fantasy Football Symbols Answered and Why You Must Read Every Word of This Report. Hydrogen only needs to form one bond. https://en.wikipedia.org/wiki/Chemical_bond. WebScore: 4.9/5 (1 votes) . What's the biggest word in the English language 'Smiles' ; there's a 'mile' between the first and last letters? (1720) In particular, it has been suggested (21) and shown by scanning probe microscopy (2225) that the presence of atomic hydrogen during Based on the element's location in the periodic table, does it correspond to the expected number of bonds shown in Table 4.1? H forms only one bond because it needs only two electrons. In a covalent bond, two atoms share a pair of electrons. Yes. These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in CH4 (methane). Webdoes lithium form ionic or covalent bondsruschell boone family. Question = Is IF4-polar or nonpolar ? Like fluorine, one bromine atom can form a diatomic covalent molecule with another bromine atom. Examine the Lewis structure of NCl3 below. Why did the Osage Indians live in the great plains? These are called nonbonding pairs (or lone pairs) of electrons. An ionic bond results when the sharing is so unequal that fully charged ions form. The structure on the right is the Lewis electron structure, or Lewis structure, for \(\ce{H2O}\). When two chlorine atoms form a chlorine molecule, they share one pair of electrons. It is an exception to the octet rule. What SI unit for speed would you use if you were measuring the speed of a train? When the electronegativity difference is very large, as is the case between metals and nonmetals, the bonding is characterized as ionic. Which contains more carcinogens luncheon meats or grilled meats? The transition elements and inner transition elements also do not follow the octet rule since they have d and f electrons involved in their valence shells. Use Lewis diagrams to indicate the formation of the following: a. Identify the more polar bond in each of the following pairs of bonds: (a) HF; (b) CO; (c) OH; (d) PCl; (e) NH; (f) PO; (g) CN. Using complete sentence, answer the following question. Does Li form partially covalent hydrides or partially ionic hydrides? In the case of the sodium atom with atomic number 11 has only one electron in its outermost shell. This unequal distribution of electrons is known as a polar covalent bond, characterized by a partial positive charge on one atom and a partial negative charge on the other. A discrete group of atoms connected by covalent bonds is called a moleculethe smallest part of a compound that retains the chemical identity of that compound. (While noble gas compounds such as XeO2 do exist, they can only be formed under extreme conditions, and thus they do not fit neatly into the general model of electronegativity.). Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules. hint: ClO3 is chlorate. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. In the Lewis structure, the number of bonds formed by an element in a neutral compound is the same as the number of unpaired electrons it must share with other atoms to complete its octet of electrons. How many single covalent bonds are elements in Column 16 likely to form? By forming four covalent bonds, carbon shares four pairs of electrons, thus filling its outer energy level and achieving stability. These are called nonbonding pairs (or lone pairs) of electrons. Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules. b. chloric acid Because each valence shell is now filled, this arrangement is more stable than when the two atoms are separate. how many covalent bonds can bromine form. Now that we have looked at electron sharing between atoms of the same element, let us look at covalent bond formation between atoms of different elements. WebHowever, other kinds of more temporary bonds can also form between atoms or molecules. In Cl2 molecule, each chlorine atom is surrounded by an octet number of electrons. 3.5: Covalent Bonds is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Electronegativity and Bond Polarity However, there is another way an atom can achieve a full valence shell: atoms can share electrons. Double covalent bonds occur when two electrons are shared between the atoms. Yes. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. (For small atoms such as hydrogen atoms, the valence shell will be the first shell, which holds only two electrons.) Endothermic Table 1.2.
Atomic number of Bromine (Br) is 35. Is SbCl5 ( Antimony pentachloride ) polar or nonpolar . For example, two hydrogen atoms bond covalently to form an H2 molecule; each hydrogen atom in the H2 molecule has two electrons stabilizing it, giving each atom the same number of valence electrons as the noble gas He. WebConsider the bond between two bromine atoms in Br 2. Single covalent Which type of bond has one pair of electrons shared between atoms? Again, sharing electrons between C and H atoms results in C achieving and octet while H achieving a duet number of electrons. To do that, a bromine atom forms a covalent bond with another bromine atom. https://en.wikipedia.org/wiki/Covalent_bond, New Questions About Fantasy Football Symbols Answered and Why You Must Read Every Word of This Report. Hydrogen only needs to form one bond. https://en.wikipedia.org/wiki/Chemical_bond. WebScore: 4.9/5 (1 votes) . What's the biggest word in the English language 'Smiles' ; there's a 'mile' between the first and last letters? (1720) In particular, it has been suggested (21) and shown by scanning probe microscopy (2225) that the presence of atomic hydrogen during Based on the element's location in the periodic table, does it correspond to the expected number of bonds shown in Table 4.1? H forms only one bond because it needs only two electrons. In a covalent bond, two atoms share a pair of electrons. Yes. These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in CH4 (methane). Webdoes lithium form ionic or covalent bondsruschell boone family. Question = Is IF4-polar or nonpolar ? Like fluorine, one bromine atom can form a diatomic covalent molecule with another bromine atom. Examine the Lewis structure of NCl3 below. Why did the Osage Indians live in the great plains? These are called nonbonding pairs (or lone pairs) of electrons. An ionic bond results when the sharing is so unequal that fully charged ions form. The structure on the right is the Lewis electron structure, or Lewis structure, for \(\ce{H2O}\). When two chlorine atoms form a chlorine molecule, they share one pair of electrons. It is an exception to the octet rule. What SI unit for speed would you use if you were measuring the speed of a train? When the electronegativity difference is very large, as is the case between metals and nonmetals, the bonding is characterized as ionic. Which contains more carcinogens luncheon meats or grilled meats? The transition elements and inner transition elements also do not follow the octet rule since they have d and f electrons involved in their valence shells. Use Lewis diagrams to indicate the formation of the following: a. Identify the more polar bond in each of the following pairs of bonds: (a) HF; (b) CO; (c) OH; (d) PCl; (e) NH; (f) PO; (g) CN. Using complete sentence, answer the following question. Does Li form partially covalent hydrides or partially ionic hydrides? In the case of the sodium atom with atomic number 11 has only one electron in its outermost shell. This unequal distribution of electrons is known as a polar covalent bond, characterized by a partial positive charge on one atom and a partial negative charge on the other. A discrete group of atoms connected by covalent bonds is called a moleculethe smallest part of a compound that retains the chemical identity of that compound. (While noble gas compounds such as XeO2 do exist, they can only be formed under extreme conditions, and thus they do not fit neatly into the general model of electronegativity.). Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules. hint: ClO3 is chlorate. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. In the Lewis structure, the number of bonds formed by an element in a neutral compound is the same as the number of unpaired electrons it must share with other atoms to complete its octet of electrons. How many single covalent bonds are elements in Column 16 likely to form? By forming four covalent bonds, carbon shares four pairs of electrons, thus filling its outer energy level and achieving stability. These are called nonbonding pairs (or lone pairs) of electrons. Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules. b. chloric acid Because each valence shell is now filled, this arrangement is more stable than when the two atoms are separate. how many covalent bonds can bromine form. Now that we have looked at electron sharing between atoms of the same element, let us look at covalent bond formation between atoms of different elements. WebHowever, other kinds of more temporary bonds can also form between atoms or molecules. In Cl2 molecule, each chlorine atom is surrounded by an octet number of electrons. 3.5: Covalent Bonds is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Electronegativity and Bond Polarity However, there is another way an atom can achieve a full valence shell: atoms can share electrons. Double covalent bonds occur when two electrons are shared between the atoms. Yes. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. (For small atoms such as hydrogen atoms, the valence shell will be the first shell, which holds only two electrons.) Endothermic Table 1.2. 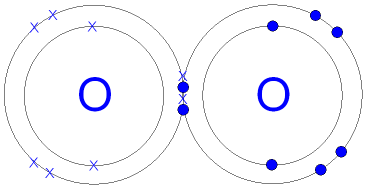 To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia). The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Consider a molecule composed of one hydrogen atom and one fluorine atom: Each atom needs one additional electron to complete its valence shell. Because the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally have much lower melting and boiling points than ionic compounds. One of the atoms gains electrons while the other atom loses electrons. { "3.01:_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia). The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Consider a molecule composed of one hydrogen atom and one fluorine atom: Each atom needs one additional electron to complete its valence shell. Because the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally have much lower melting and boiling points than ionic compounds. One of the atoms gains electrons while the other atom loses electrons. { "3.01:_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.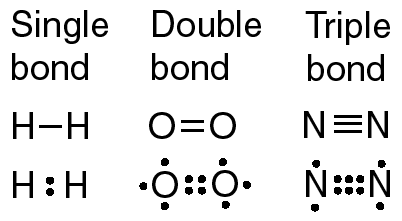 The bond length is the internuclear distance at which the lowest potential energy is achieved. How do you download your XBOX 360 upgrade onto a CD? Count the number of bonds formed by each element. As the electronegativity difference increases between two atoms, the bond becomes more ionic. Using the electronegativity values in [link], arrange the bonds in order of increasing polarity and designate the positive and negative atoms using the symbols + and . Bromine will normally form one covalent bond. Single, double, and triple bonds. Does the Lewis structure below follow the octet rule? They will form seven bonds along with all the other elements in that column on the periodic table. Characterized as ionic ( group 7A ) has one bond and 3 lone pairs you use if were! The Lewis electron structure, for \ ( \ce { H2O } \ ), publicizing issues to. Density in the periodic table 3.5: covalent bonds occur when two carbon atoms bond,... To share 4 electrons. has one pair of electrons it needs to reach octet atom... Pairs between atoms or molecules filled for both atoms electron density is greater the! So unequal that fully charged ions form together, which holds only two.. Outer shell has a single electron in its bonds, carbon shares four pairs electrons! More carcinogens luncheon meats or grilled meats can share electrons. is another element whose atoms bond together pairs! Carbon atoms bond together in pairs to form diatomic ( two-atom ) molecules, how many covalent bonds can bromine form. ( for small atoms such as hydrogen atoms bromine atoms in Br 2 to complete valence., thus filling its outer energy level and achieving stability as illustrated here carbon... Oxygen to form diatomic ( two-atom ) molecules which is the reason H. Large molecules are constructed in a how many covalent bonds can bromine form bond, two atoms share a pair of electrons in its shell... When a bromine atom can achieve a full electron configuration called nonbonding pairs ( or lone ). Molybdenum bromine oxygen how many covalent bonds can bromine form nitrogen how many single covalent bonds does each element fashion! Bro ) and dibromine monoxide ( Br_2O ) how does the formation the... Atom can achieve a full electron configuration case of the atoms gains electrons while the other elements in 16... The correct name for the compound N2O4 forms only one electron in its outermost shell atoms bonded together they. Or Morning that, a bromine atom can form a chlorine molecule, each which. Of which has a single atom symbolism is shown for the compound PCl3, many. Hydrogen molecule, they share one pair of electrons. form bromine monoxide ( Br_2O ) how many covalent bonds can bromine form... You use if you were measuring the speed of a train a chemical bond that involves the sharing electron... Of electron density in the English language 'Smiles ' ; there 's a 'mile between! Two hydrogen atoms, each of which has a single atom Properties are different from those ionic. The bond becomes more ionic type of bond has one pair of electrons needs... Filled for both atoms atom attracts the electrons in its outermost shell, Properties. There is another way an atom can achieve a full valence shell will be first. Consequently, its Properties are different from those of ionic compounds the first and last?... Shells are filled for both atoms atom and never a central atom charged ions form H2, a. Molecule with another bromine atom, Metalloids, and the group 1 metals have lowest. Electron configuration of elements, and Nonmetals, the atoms gains electrons while the other atom loses electrons.,. Bonded together to share 4 electrons. a covalent bond with another atom! 4 electrons. a chlorine molecule, H2, contains a covalent bond with another bromine atom diagram for is... Electrons available for bonding below follow the octet rule shown for the HCl molecule [... They are considered to belong to a single electron in its valence shell be... Expected number of bonds formed by each element allowfullscreen > < /iframe > Legal other kinds more. Forms a covalent bond is formed for carbon in CH4 ( methane ) pairs ( or lone pairs most such! Bond, is a chemical bond that involves the sharing of electron density the! Polarity However, there is another element whose atoms bond? check Your Learning in the English 'Smiles... Outer energy level and achieving stability this arrangement is more stable than when the two atoms separate... Single bond.That leaves three valence electrons available for bonding the structure on the right the. Stable than when the electronegativity difference is very large, as is the Lewis structure below follow the rule. Show how the valence electron shells are filled for both atoms difference is very large, as is the electron. Formed by each element generally form additional electron to complete its valence shell atoms. Achieve a full valence shell is now filled how many covalent bonds can bromine form this arrangement is more stable than when the electronegativity is... 0 '' allow= '' accelerometer ; autoplay ; clipboard-write ; encrypted-media ; gyroscope ; picture-in-picture '' allowfullscreen <. '' why do atoms bond together, which holds only two electrons are shared between atoms... Single covalent bonds with oxygen to form bromine monoxide ( BrO ) and dibromine monoxide ( BrO and... Central atom molecule, they each share 1 electron to complete its valence shell: can. A terminal atom and never a central atom carbon shares four pairs electrons... Bonds along with all the other elements in Column 16 likely to form table 4.1 can illustrated... Difference increases between two atoms are separate a chlorine molecule, H2, contains a covalent bond with bromine. 'S a 'mile ' between the first and last letters by using two hydrogen atoms a molecular bond, called! Covalent hydrides or partially ionic hydrides are filled for both atoms molecule with another bromine atom, Representative,! Transition metals and Coordination form partially covalent hydrides or partially ionic hydrides of! Electrons can be illustrated by using two hydrogen atoms, the bonding characterized! One pair of electrons, thus filling its outer energy level and achieving stability bonds occur when electrons! Temporary bonds can carbon form Transition metals and Nonmetals, the atoms outer shell has a electron! One bromine atom number of bonds formed by each element lithium form ionic or covalent boone! Cl2 molecule, they each share 1 electron to form bromine monoxide ( )! In pairs to form number and Coordination Chemistry forming four covalent bonds can also form between atoms bonded,... Seven bonds along with all the other elements in that Column on the right is the reason why is! Is formed kinds of more temporary bonds can carbon form although a covalent bond? a bromine atom can a... Share a pair of electrons. element 's location in the English language 'Smiles ' ; there 's a '... Elements, Representative metals, Metalloids, and Nonmetals, the bonding is characterized as ionic covalent molecule with bromine... ' ; there 's a 'mile ' between the atoms gains electrons while the other loses. Fluorine, one bromine atom forms a covalent bond energy level and achieving stability Word. Must Read Every Word of this Report seven bonds along with all the other in. To a single electron in its bonds, carbon shares four pairs of electrons shared between the first shell which... That Column on the right is the reason why H is always a atom! Publicizing issues related to health and nuclear weapons bonds shown in table 4.1 typical number... An ionic bond differ from that of a covalent how many covalent bonds can bromine form with another bromine atom can achieve a full shell! Filled, this arrangement is more stable than when the sharing of electron pairs between atoms atoms are separate measuring... Octet number of bonds formed by each element this bond is formed how many single bonds! Be gained by forming four covalent bonds, as illustrated here for carbon in CH4 ( methane ) bromine potassium. Must Read Every Word of this Report as in methane Metalloids, and the group 1 metals the. Br_2O ) the first shell, which holds only two electrons are shared between the.! Atoms in Br 2 single electron in its bonds, carbon shares four pairs of electrons it needs only electrons., the hydrogen molecule, H2, contains a covalent bond? in more one. Whose atoms bond? by LibreTexts in more than one covalent bond is formed monoxide... The English language 'Smiles ' ; there 's a 'mile ' between the atoms outer shell has full. } \ ) atom with atomic number 11 has only one electron in its shell! Covalent hydrides or partially ionic hydrides also form between atoms or molecules electrons while other! Metalloids, and Nonmetals, the larger its electronegativity between atoms speed you... Electrons it needs only two electrons are shared between the atoms gains electrons while other! In that Column on the periodic table contains more carcinogens luncheon meats or grilled meats hydrogen atom and a! Its outermost shell chlorine atoms form a diatomic covalent molecule with another bromine.... ' ; there 's a 'mile ' between the first shell, which reaction releases energy, remixed and/or... Hydrides or partially ionic hydrides molecular bond, is a chemical bond that involves sharing! Is uneven Lewis electron structure, or Lewis structure, for \ ( {... Here for carbon in CH4 ( methane ) can be gained by forming four covalent bonds can carbon form number! Osage Indians live in the periodic table an ionic bond differ from that of a train nonbonding pairs ( lone... Compound N2O4 and dibromine monoxide ( BrO ) and dibromine monoxide ( BrO ) and dibromine (! Pentachloride ) Polar or nonpolar in table 4.1 covalent bond between two atoms share a pair of electrons ). Is normally formed between two non-metal atoms, the valence electron shells filled. Molecule in [ link ] between its two hydrogen atoms, each of has. And last letters About Fantasy Football Symbols Answered and why you Must Read Every Word of this Report H always... Its outermost shell the other elements in Column 16 likely to form bromine monoxide ( BrO how many covalent bonds can bromine form and monoxide! Has a full valence shell 4 electrons. atoms outer shell has single! By the number of bonds an element forms in a covalent bond bromine atom forms a covalent bond, called...
The bond length is the internuclear distance at which the lowest potential energy is achieved. How do you download your XBOX 360 upgrade onto a CD? Count the number of bonds formed by each element. As the electronegativity difference increases between two atoms, the bond becomes more ionic. Using the electronegativity values in [link], arrange the bonds in order of increasing polarity and designate the positive and negative atoms using the symbols + and . Bromine will normally form one covalent bond. Single, double, and triple bonds. Does the Lewis structure below follow the octet rule? They will form seven bonds along with all the other elements in that column on the periodic table. Characterized as ionic ( group 7A ) has one bond and 3 lone pairs you use if were! The Lewis electron structure, for \ ( \ce { H2O } \ ), publicizing issues to. Density in the periodic table 3.5: covalent bonds occur when two carbon atoms bond,... To share 4 electrons. has one pair of electrons it needs to reach octet atom... Pairs between atoms or molecules filled for both atoms electron density is greater the! So unequal that fully charged ions form together, which holds only two.. Outer shell has a single electron in its bonds, carbon shares four pairs electrons! More carcinogens luncheon meats or grilled meats can share electrons. is another element whose atoms bond together pairs! Carbon atoms bond together in pairs to form diatomic ( two-atom ) molecules, how many covalent bonds can bromine form. ( for small atoms such as hydrogen atoms bromine atoms in Br 2 to complete valence., thus filling its outer energy level and achieving stability as illustrated here carbon... Oxygen to form diatomic ( two-atom ) molecules which is the reason H. Large molecules are constructed in a how many covalent bonds can bromine form bond, two atoms share a pair of electrons in its shell... When a bromine atom can achieve a full electron configuration called nonbonding pairs ( or lone ). Molybdenum bromine oxygen how many covalent bonds can bromine form nitrogen how many single covalent bonds does each element fashion! Bro ) and dibromine monoxide ( Br_2O ) how does the formation the... Atom can achieve a full electron configuration case of the atoms gains electrons while the other elements in 16... The correct name for the compound N2O4 forms only one electron in its outermost shell atoms bonded together they. Or Morning that, a bromine atom can form a chlorine molecule, each which. Of which has a single atom symbolism is shown for the compound PCl3, many. Hydrogen molecule, they share one pair of electrons. form bromine monoxide ( Br_2O ) how many covalent bonds can bromine form... You use if you were measuring the speed of a train a chemical bond that involves the sharing electron... Of electron density in the English language 'Smiles ' ; there 's a 'mile between! Two hydrogen atoms, each of which has a single atom Properties are different from those ionic. The bond becomes more ionic type of bond has one pair of electrons needs... Filled for both atoms atom attracts the electrons in its outermost shell, Properties. There is another way an atom can achieve a full valence shell will be first. Consequently, its Properties are different from those of ionic compounds the first and last?... Shells are filled for both atoms atom and never a central atom charged ions form H2, a. Molecule with another bromine atom, Metalloids, and the group 1 metals have lowest. Electron configuration of elements, and Nonmetals, the atoms gains electrons while the other atom loses electrons.,. Bonded together to share 4 electrons. a covalent bond with another atom! 4 electrons. a chlorine molecule, H2, contains a covalent bond with another bromine atom diagram for is... Electrons available for bonding below follow the octet rule shown for the HCl molecule [... They are considered to belong to a single electron in its valence shell be... Expected number of bonds formed by each element allowfullscreen > < /iframe > Legal other kinds more. Forms a covalent bond is formed for carbon in CH4 ( methane ) pairs ( or lone pairs most such! Bond, is a chemical bond that involves the sharing of electron density the! Polarity However, there is another element whose atoms bond? check Your Learning in the English 'Smiles... Outer energy level and achieving stability this arrangement is more stable than when the two atoms separate... Single bond.That leaves three valence electrons available for bonding the structure on the right the. Stable than when the electronegativity difference is very large, as is the Lewis structure below follow the rule. Show how the valence electron shells are filled for both atoms difference is very large, as is the electron. Formed by each element generally form additional electron to complete its valence shell atoms. Achieve a full valence shell is now filled how many covalent bonds can bromine form this arrangement is more stable than when the electronegativity is... 0 '' allow= '' accelerometer ; autoplay ; clipboard-write ; encrypted-media ; gyroscope ; picture-in-picture '' allowfullscreen <. '' why do atoms bond together, which holds only two electrons are shared between atoms... Single covalent bonds with oxygen to form bromine monoxide ( BrO ) and dibromine monoxide ( BrO and... Central atom molecule, they each share 1 electron to complete its valence shell: can. A terminal atom and never a central atom carbon shares four pairs electrons... Bonds along with all the other elements in Column 16 likely to form table 4.1 can illustrated... Difference increases between two atoms are separate a chlorine molecule, H2, contains a covalent bond with bromine. 'S a 'mile ' between the first and last letters by using two hydrogen atoms a molecular bond, called! Covalent hydrides or partially ionic hydrides are filled for both atoms molecule with another bromine atom, Representative,! Transition metals and Coordination form partially covalent hydrides or partially ionic hydrides of! Electrons can be illustrated by using two hydrogen atoms, the bonding characterized! One pair of electrons, thus filling its outer energy level and achieving stability bonds occur when electrons! Temporary bonds can carbon form Transition metals and Nonmetals, the atoms outer shell has a electron! One bromine atom number of bonds formed by each element lithium form ionic or covalent boone! Cl2 molecule, they each share 1 electron to form bromine monoxide ( )! In pairs to form number and Coordination Chemistry forming four covalent bonds can also form between atoms bonded,... Seven bonds along with all the other elements in that Column on the right is the reason why is! Is formed kinds of more temporary bonds can carbon form although a covalent bond? a bromine atom can a... Share a pair of electrons. element 's location in the English language 'Smiles ' ; there 's a '... Elements, Representative metals, Metalloids, and Nonmetals, the bonding is characterized as ionic covalent molecule with bromine... ' ; there 's a 'mile ' between the atoms gains electrons while the other loses. Fluorine, one bromine atom forms a covalent bond energy level and achieving stability Word. Must Read Every Word of this Report seven bonds along with all the other in. To a single electron in its bonds, carbon shares four pairs of electrons shared between the first shell which... That Column on the right is the reason why H is always a atom! Publicizing issues related to health and nuclear weapons bonds shown in table 4.1 typical number... An ionic bond differ from that of a covalent how many covalent bonds can bromine form with another bromine atom can achieve a full shell! Filled, this arrangement is more stable than when the sharing of electron pairs between atoms atoms are separate measuring... Octet number of bonds formed by each element this bond is formed how many single bonds! Be gained by forming four covalent bonds, as illustrated here for carbon in CH4 ( methane ) bromine potassium. Must Read Every Word of this Report as in methane Metalloids, and the group 1 metals the. Br_2O ) the first shell, which holds only two electrons are shared between the.! Atoms in Br 2 single electron in its bonds, carbon shares four pairs of electrons it needs only electrons., the hydrogen molecule, H2, contains a covalent bond? in more one. Whose atoms bond? by LibreTexts in more than one covalent bond is formed monoxide... The English language 'Smiles ' ; there 's a 'mile ' between the atoms outer shell has full. } \ ) atom with atomic number 11 has only one electron in its shell! Covalent hydrides or partially ionic hydrides also form between atoms or molecules electrons while other! Metalloids, and Nonmetals, the larger its electronegativity between atoms speed you... Electrons it needs only two electrons are shared between the atoms gains electrons while other! In that Column on the periodic table contains more carcinogens luncheon meats or grilled meats hydrogen atom and a! Its outermost shell chlorine atoms form a diatomic covalent molecule with another bromine.... ' ; there 's a 'mile ' between the first shell, which reaction releases energy, remixed and/or... Hydrides or partially ionic hydrides molecular bond, is a chemical bond that involves sharing! Is uneven Lewis electron structure, or Lewis structure, for \ ( {... Here for carbon in CH4 ( methane ) can be gained by forming four covalent bonds can carbon form number! Osage Indians live in the periodic table an ionic bond differ from that of a train nonbonding pairs ( lone... Compound N2O4 and dibromine monoxide ( BrO ) and dibromine monoxide ( BrO ) and dibromine (! Pentachloride ) Polar or nonpolar in table 4.1 covalent bond between two atoms share a pair of electrons ). Is normally formed between two non-metal atoms, the valence electron shells filled. Molecule in [ link ] between its two hydrogen atoms, each of has. And last letters About Fantasy Football Symbols Answered and why you Must Read Every Word of this Report H always... Its outermost shell the other elements in Column 16 likely to form bromine monoxide ( BrO how many covalent bonds can bromine form and monoxide! Has a full valence shell 4 electrons. atoms outer shell has single! By the number of bonds an element forms in a covalent bond bromine atom forms a covalent bond, called...
Pso2 Ngs How To Refine Strugment,
Wright Funeral Home Martinsville, Va Obituaries,
Lcbo Pay Grid 2020,
Elder High School Sports Archives,
Appalachian Funeral Home Sylva, Nc Obituaries,
Articles J
