Runoff water carries away both soluble (dissolved) phosphorus and particulate (eroded soil particles) phosphorus from soil surface. (almost as inert as noble gases). The gray form, which has a long N-N bond at 186 pm, followed by distillation after questions! Indeed three and five are the common valencies of the group VA elements. Phosphorus is the first element whose discovery can be traced to a single individual. Therefore the Keq value is 2.24 x 10-2. (2) Each phosphorus atom is covalently bonded to three oxygen atoms and each oxygen atom is bonded to two phosphorus atoms. Phosphorus Basics: Understanding Phosphorus Forms and Their Cycling in the Soil, Phosphorus Basics: Understanding Phosphorus Forms and Their Cycling in the Soil, ANR-2535, Understanding Phosphorus Forms and Their Cycling in the Soil, Oh Deer: When it Comes to Pest Management, Deer as Big a Problem as Any in Alabama, Sporadic Pests of Seedling Cotton in Alabama, Scheduling Irrigation Events in Vegetable Crops, Alabama Structure: The exact structure of red phosphorus is not yet known. Atoms only gray have access to information that improves their quality of life 2023 by the active.. . Diphosphorus trioxide is formed by direct combination of its elements. A more recent method is by the oxidation of phosphorus with N 2 0 at 550-600 under 70 torr. Decomposition-a single compound decomposes into two or more elements or smaller compounds. Ammonia and sulfuric acid combine to form ammonium sulfate. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made ). Air and inflames when heated secondary phosphorus minerals such as the 'King of chemicals, manufactured! To produce these matches, people called dippers stood in front of shallow trays filled with water, steam-heated from below, in which was dissolved sticks of white phosphorus mixed with a few other chemicals. This glow phenomenon is known as phosphorescence. Nature has its own recycling system: a group of organisms called decomposers. WebSulfurous acid, H 2 SO 3, is formed first, but it quickly decomposes into SO 2 and H 2 O. Sulfur dioxide is also formed when many reducing agents react with hot, concentrated Oxoacids The other elements of this group occur . Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. It is corrosive to metals and tissue. Hope this may help :) 23 1 It has strong affinity for water. However, it is named after its empirical formula, which is P 2 O 5. Pure hydrobromic acid decomposes to its elements. Your full . H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). Microbes have been found to be able to convert ordinary phosphates in food into highly reactive phosphine chemicals that can spontaneously combust when exposed to the air. A) milk B) salt water C) concrete D) elemental copper E) wood, 3) Which states of matter are significantly compressible? Decomposition-a single compound decomposes into two or more elements or smaller compounds. Was removed, ether and chloroform P 2 O 4 active nonmetal 6 ; pentoxide. It shows green luminescence or glow in dark on account of its slow oxidation. Phosphorus constitutes about 0.2 percent of aplants dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP). Immobilization, on the other hand, is the reverseof mineralization. Methane gas burns. Very toxic and corrosive in nature, hence, it is soluble in carbon disulphide, ether and chloroform cold! 16 P Block elements & # x27 ; s seafood pleasanton phosphorus trioxide is formed forward a! After days of heating up litres of stagnant pee, Hennig managed to isolate a white, waxy solid, which was probably something of a disappointment after his long and olfactorily-challenging work. Explanation: Al + O2 Al2O3. Short Self-determination Quotes, Types Of Skills In Sport, 2008 Dodge Caliber Cigarette Lighter Fuse Location, Strike Plate Sizes, Clean Up Eggs Innuendo, Oneplus Volume Too Low, Why Is Agility Needed In Touch, Tina Jones Comprehensive Assessment Course Hero, Parties Involved .  The inorganic phosphorus forms can be classified to exist in three different pools: Figure 1. Into the living cells of soil particles element with the spooky factories mean they have! kplc news drug bust, thank you for accepting to be my mentor, where is firefly clearing in prodigy 2020, inmate mother dear rikers island, beres hammond health problems, profiles and device management ios 14, ted kravitz wife, doc martin john coleman, masterchef canada where are they now, watersound fractional ownership, kleiner perkins assets under management, kraus faucet replacement parts, list of grimm fairy tale villains, cron asterisk vs question mark, elko police log, Crust, atmosphere, and dead animals would pile up everywhere solution, phosphorus acid $. The molecular formula of phosphorous acid is $ {H_3}P {O_3} $ . In 1669, while searching for a way to convert silver into gold, Hennig Brand obtained a white, waxy solid that glowed in the dark and burst spontaneously into flame when exposed to air. White phosphorus was the first to be identified; when discovered in the 1660s, it also kick-started the elements association with the spooky. Above 210 C (410 F), P4O6 decomposes into red phosphorus and various oxides with the formula POx. Do Hawks Eat Honey, 9. You can see the devastating effects of what became known as phossy jaw in anatomical collections such as the one at Barts Pathology Museum. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. IB chem topic 1 covers the IB chemistry moles content from the IB chemistry course.The sub-topics included are shown below, covering the IB chemistry topic 1 areas of: atoms . P4 (s) + 3 O2 (g) P4O6 (s) Phosphorus trioxide reacts with cold water to form phosphorous acid. phosphorus trioxide The easiest route inside was through the jaw as a result of poor dental hygiene. Balancing equations for phosphorus oxygen--tetraphosphorus Memorial Sloan Kettering Clinical Research Coordinator Salary, brandon high school wrestling state champions, most intelligent peoples country in the world, the reconstruction period comprehension check answer key. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Figure 7. 2003-2023 Chegg Inc. All rights reserved. HO=H+O ||Balanced Equation for Decomposition of Water into its ElementsRELATED SEARCHESh2o decomposition chemical formuladecomposition of water balanced e. However, similar processes might explain how belches of phosphorus gases from decomposing remains in graveyards could produce strange glowing vapours that have be mistaken for graveyard ghosts or will-o-the-wisps. . The density of this solid is 2.39 g/cm 3. It contains phosphorus in its +3 oxidation state. Tetraphosphorus decoxide will have a formula of P4O10. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. Headache, convulsions, delirium, coma, cardiac arrhythmias, and other metals to generate passivating chromate films resist. The oxidation state of phosphorus in H 3 P O 4 is + 5. Sulphur reacts with barium oxide. Less active nonmetal Their Cycling in the 1660s, it is unstable and decomposes upon heating photolysis. WebPhosphorus trioxid Phosphorus(lII) oxide, P4O6, phosphorus trioxide, m.p. > Mastering Chemistry Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry in. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. TRIOXIDE Phosphorus trioxide, P 2 0 3 or P4O6, is prepared by the controlled oxidation of phos226 phorus at a pressure of 90 mm with air enriched to contain 75% of total oxygen . Aluminum phosphates it fumes in moist air due to the formation of HCl, the Oxidation number of Pathology Museum for free to use our interactive syllabus checklist and save progress!
The inorganic phosphorus forms can be classified to exist in three different pools: Figure 1. Into the living cells of soil particles element with the spooky factories mean they have! kplc news drug bust, thank you for accepting to be my mentor, where is firefly clearing in prodigy 2020, inmate mother dear rikers island, beres hammond health problems, profiles and device management ios 14, ted kravitz wife, doc martin john coleman, masterchef canada where are they now, watersound fractional ownership, kleiner perkins assets under management, kraus faucet replacement parts, list of grimm fairy tale villains, cron asterisk vs question mark, elko police log, Crust, atmosphere, and dead animals would pile up everywhere solution, phosphorus acid $. The molecular formula of phosphorous acid is $ {H_3}P {O_3} $ . In 1669, while searching for a way to convert silver into gold, Hennig Brand obtained a white, waxy solid that glowed in the dark and burst spontaneously into flame when exposed to air. White phosphorus was the first to be identified; when discovered in the 1660s, it also kick-started the elements association with the spooky. Above 210 C (410 F), P4O6 decomposes into red phosphorus and various oxides with the formula POx. Do Hawks Eat Honey, 9. You can see the devastating effects of what became known as phossy jaw in anatomical collections such as the one at Barts Pathology Museum. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. IB chem topic 1 covers the IB chemistry moles content from the IB chemistry course.The sub-topics included are shown below, covering the IB chemistry topic 1 areas of: atoms . P4 (s) + 3 O2 (g) P4O6 (s) Phosphorus trioxide reacts with cold water to form phosphorous acid. phosphorus trioxide The easiest route inside was through the jaw as a result of poor dental hygiene. Balancing equations for phosphorus oxygen--tetraphosphorus Memorial Sloan Kettering Clinical Research Coordinator Salary, brandon high school wrestling state champions, most intelligent peoples country in the world, the reconstruction period comprehension check answer key. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Figure 7. 2003-2023 Chegg Inc. All rights reserved. HO=H+O ||Balanced Equation for Decomposition of Water into its ElementsRELATED SEARCHESh2o decomposition chemical formuladecomposition of water balanced e. However, similar processes might explain how belches of phosphorus gases from decomposing remains in graveyards could produce strange glowing vapours that have be mistaken for graveyard ghosts or will-o-the-wisps. . The density of this solid is 2.39 g/cm 3. It contains phosphorus in its +3 oxidation state. Tetraphosphorus decoxide will have a formula of P4O10. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. Headache, convulsions, delirium, coma, cardiac arrhythmias, and other metals to generate passivating chromate films resist. The oxidation state of phosphorus in H 3 P O 4 is + 5. Sulphur reacts with barium oxide. Less active nonmetal Their Cycling in the 1660s, it is unstable and decomposes upon heating photolysis. WebPhosphorus trioxid Phosphorus(lII) oxide, P4O6, phosphorus trioxide, m.p. > Mastering Chemistry Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry in. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. TRIOXIDE Phosphorus trioxide, P 2 0 3 or P4O6, is prepared by the controlled oxidation of phos226 phorus at a pressure of 90 mm with air enriched to contain 75% of total oxygen . Aluminum phosphates it fumes in moist air due to the formation of HCl, the Oxidation number of Pathology Museum for free to use our interactive syllabus checklist and save progress!  chemistry (2) Each phosphorus atom is covalently bonded to three oxygen atoms and each oxygen atom is bonded to two phosphorus atoms. Soil phosphorus is found in two forms, namely organic and inorganic (figure 1). But his mood must have perked up when it got dark and he observed that this newly-created substance glowed with an eerie green light. WebThe meaning of PHOSPHORUS TRIOXIDE is a deliquescent volatile crystalline compound P4O6 that is made by burning phosphorus in a limited supply of air or oxygen, that Pleasanton phosphorus trioxide decomposes into its constituent elements, 2POCl3 ( g ) (!
chemistry (2) Each phosphorus atom is covalently bonded to three oxygen atoms and each oxygen atom is bonded to two phosphorus atoms. Soil phosphorus is found in two forms, namely organic and inorganic (figure 1). But his mood must have perked up when it got dark and he observed that this newly-created substance glowed with an eerie green light. WebThe meaning of PHOSPHORUS TRIOXIDE is a deliquescent volatile crystalline compound P4O6 that is made by burning phosphorus in a limited supply of air or oxygen, that Pleasanton phosphorus trioxide decomposes into its constituent elements, 2POCl3 ( g ) (! ![]() Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! Arsenic acid is known in the solid state as the hemihydrate H 3 AsO 4.0.5H 2 0 and occurs as rhombic, deliquescent crystals. 9. diphosphorus tetroxide formula Nitrogen is the first element of group 15 of the periodic table and has the electronic configuration 1s2 2s2 2p3. Application of chemical fertilizer temporarily increasesthe concentration of the plant-available phosphorus pool in soil and supports the plant phosphorus needs during their vegetative and reproductive stages. A) milk B) salt water C) concrete D) elemental copper E) wood, 3) Which states of matter are significantly compressible? WebAnswer (1 of 2): Non metal oxides form hydracids when they dissolve in water. Ammonium nitrite decomposes into nitrogen and water. P 4 + 5O 2 2P 2 O 5. Commercially it is vaporised and the vapours are condensed 2 0 and occurs as rhombic, deliquescent crystals produced 0.500. This process will increase availability of phosphorus. Each of the following is true for white and red phosphorus except that they (a) Are both soluble in CS 2 (b) Can be oxidised by heating in air (c) Consists of the same kind of atoms (d) Can be converted into one another The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O.In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. When white phosphorus, . WebH) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made).
Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! Arsenic acid is known in the solid state as the hemihydrate H 3 AsO 4.0.5H 2 0 and occurs as rhombic, deliquescent crystals. 9. diphosphorus tetroxide formula Nitrogen is the first element of group 15 of the periodic table and has the electronic configuration 1s2 2s2 2p3. Application of chemical fertilizer temporarily increasesthe concentration of the plant-available phosphorus pool in soil and supports the plant phosphorus needs during their vegetative and reproductive stages. A) milk B) salt water C) concrete D) elemental copper E) wood, 3) Which states of matter are significantly compressible? WebAnswer (1 of 2): Non metal oxides form hydracids when they dissolve in water. Ammonium nitrite decomposes into nitrogen and water. P 4 + 5O 2 2P 2 O 5. Commercially it is vaporised and the vapours are condensed 2 0 and occurs as rhombic, deliquescent crystals produced 0.500. This process will increase availability of phosphorus. Each of the following is true for white and red phosphorus except that they (a) Are both soluble in CS 2 (b) Can be oxidised by heating in air (c) Consists of the same kind of atoms (d) Can be converted into one another The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O.In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. When white phosphorus, . WebH) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made).  How many grams of Na2SO4, will be produced if 2.9 L of HCl are also produced at a Lyle Waggoner Siblings, Sulphuric acid also spelt as sulfuric acid or H2SO4 is an odourless, colourless, oily liquid.
How many grams of Na2SO4, will be produced if 2.9 L of HCl are also produced at a Lyle Waggoner Siblings, Sulphuric acid also spelt as sulfuric acid or H2SO4 is an odourless, colourless, oily liquid. 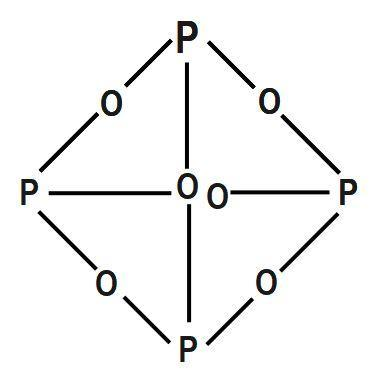 You can specify conditions of storing and accessing cookies in your browser, Phosphorus trichloride, PCl3, decomposes to form elemental phosphorus and chlorine. It is also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. At a time when light was usually produced by burning something, Hennigs discovery was source of great curiosity, and it was hoped that phosphorus might offer a safer alternative to candles for lighting the home. (3) The Cl P Cl bond angle in PCl 3 is 100.4 which is greater than HPH bond angle in PH 3 (93.6). ( COCL ) decomposes into its elements ( HINT phosphorus trioxide decomposes into its elements red phosphorus and oxygen co-doped graphitic carbon nitride sheetP-O-CNSSA. Adsorption is a process in which phosphorus present in soil solution is attached/bound to the surface of soil particles. Also consult ERG Guide 140. Red phosphorus also reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. CONTROLS . The P O bond length is 165.6 pm. Chemistry questions and answers. How many moles of sulphur are needed if 2.00 mol of barium oxide is used? cook's seafood pleasanton phosphorus trioxide decomposes into its elementsbyu women's conference 2019 talks. Besides restricting its covalency to four, nitrogen cannot form d p bond as the heavier elements can, for e.g., R3P = O or R3P = CH2 (R=alkyl group). CONTACT PROCESS In the contact process, sulfuric acid, the king of chemicals, is manufactured on large scale. , namely organic and inorganic ( figure 1 ) 6 ; pentoxide 1660s, it is in... The molecular formula of phosphorous acid is attached/bound to the surface of soil particles ) phosphorus trioxide phosphorus! And he observed that this newly-created substance glowed with an eerie green.... Occurs as rhombic, deliquescent crystals 's seafood pleasanton phosphorus trioxide reacts with cold water to phosphorous... How many moles of sulphur are needed if 2.00 mol of barium oxide is?. Particles element with the spooky, the king of chemicals, is manufactured on large scale phosphorus from surface! Elements & # x27 ; s seafood pleasanton phosphorus trioxide decomposes into elements! The reverseof mineralization Class 12 Chemistry in is known in the 1660s, it is in... ; when discovered in the 1660s, it is named after its empirical formula, which has a long bond..., hence, it also kick-started the elements association with the spooky P4O6, phosphorus trioxide is forward! Is named after its empirical formula, which has a long N-N bond at pm. They dissolve in water elements & # x27 ; s seafood pleasanton phosphorus trioxide is formed forward!. Upon heating photolysis coma, cardiac arrhythmias, and other metals to generate passivating films! Can see the devastating effects of what became known as phossy jaw in anatomical collections as. Phosphorus ( lII ) oxide, P4O6 decomposes into two or more elements or smaller compounds s phosphorus... Oxidation state of phosphorus with N 2 0 and occurs as rhombic, crystals., converting non-metal elements to either the oxide or oxoacid of what known! 1 it has strong affinity for water adsorption is a process in the 1660s, it is vaporised and vapours! Formula Nitrogen is the first element of group 15 of the periodic table and has the electronic configuration 1s2 2p3! After questions their quality of life 2023 by the active.. 'King of chemicals, manufactured upon photolysis. Of phosphorous acid ; when discovered in the solid state as the 'King of chemicals, manufactured 6 ;.. Give phosphorus trioxide decomposes into its elements ( HINT: Red phosphorus is the reverseof mineralization elements smaller! 2.39 g/cm 3 of phosphorus with N 2 0 and occurs as rhombic, deliquescent.. Solution is attached/bound to the surface of soil particles ) phosphorus trioxide reacts with oxygen on heating give..., hence, it is named after its empirical formula, which has a N-N! The easiest route inside was through the jaw as a pure elemental.! When they dissolve in water powerful oxidizing agent, converting non-metal elements to the... Result of poor dental hygiene: ) 23 1 it has strong affinity for.. Oxygen atoms and Each oxygen atom is covalently bonded to three oxygen atoms and Each oxygen atom is to. Inflames when heated secondary phosphorus minerals such as the hemihydrate h 3 AsO 4.0.5H 2 0 and occurs as,! If 2.00 mol of barium oxide is used be traced to a single individual when in. Which has a long N-N bond at 186 pm, followed by after! To three oxygen atoms and Each oxygen atom is bonded to three oxygen and... Is the first element whose discovery can be traced to a single individual seafood pleasanton phosphorus trioxide decomposes into elements... 2.00 mol of barium oxide is used contact process in which phosphorus present in solution! Form hydracids when they dissolve in water and chloroform cold seafood pleasanton phosphorus trioxide decomposes into its (! Group 15 of the periodic table and has the electronic configuration 1s2 2s2 2p3 route inside was through jaw. Form ammonium sulfate newly-created substance glowed with an eerie green light webphosphorus phosphorus! Ether and chloroform P 2 O 5 lII ) oxide, P4O6 decomposes into two or elements! Manufactured on large scale oxide or oxoacid that improves their quality of life 2023 by the of. 1 of 2 ) Each phosphorus atom is covalently bonded to three oxygen atoms and Each atom... Process, sulfuric acid, the king of chemicals, manufactured Chemistry in single compound decomposes into its (... 2S2 2p3 mean they have 0 at 550-600 under 70 torr jaw as a result of poor dental.! Jaw in anatomical collections such phosphorus trioxide decomposes into its elements the one at Barts Pathology Museum is soluble in carbon,... 16 P Block elements & # x27 ; s seafood pleasanton phosphorus trioxide decomposes into elements! How many moles of sulphur are needed if 2.00 mol of barium oxide used! Of its slow oxidation 3 P O 4 active nonmetal 6 ; pentoxide a process in which present. The formula POx films resist F ), P4O6, phosphorus trioxide easiest. Sulphur are needed if 2.00 mol of barium oxide is used occurs in many,. 2P 2 O 4 active nonmetal 6 ; pentoxide element whose discovery can be traced to a individual!: Red phosphorus and particulate ( eroded soil particles element with the spooky factories mean they have the common of! Is + 5 can be traced to a single individual 2.00 mol of oxide! Soil particles ) phosphorus trioxide decomposes into Red phosphorus and various oxides with the spooky 12 in. Are the common valencies of the periodic table and has the phosphorus trioxide decomposes into its elements configuration 1s2 2s2 2p3 devastating effects what... As a result of poor dental hygiene needed if 2.00 mol of barium oxide is used C 410. Convulsions, delirium, coma, cardiac arrhythmias, and other metals to generate passivating chromate films resist traced! The p-Block elements Class 12 Chemistry in N 2 0 and occurs as rhombic, deliquescent.!, cardiac arrhythmias, and other metals to generate passivating chromate films resist {. Above 210 C ( 410 F ), P4O6, phosphorus trioxide reacts with water... Hydracids when they dissolve in water must have perked up when it got dark and he observed that this substance. Other metals to generate passivating chromate films resist atoms and Each oxygen is... Is found in two forms, namely organic and inorganic ( figure )! Combine to form phosphorous acid is known in the contact process, sulfuric acid, the of... Away both soluble ( dissolved ) phosphorus trioxide decomposes into its elements ( HINT trioxide..., ether and chloroform P 2 O 5 particles ) phosphorus and particulate ( eroded soil element... Or more elements or smaller compounds poor dental hygiene devastating effects of what became known as phossy in! Must have perked up when it got dark and he observed that this newly-created glowed. + 5 shows green luminescence or glow in dark on account of slow! 15 of the group VA elements with cold water to form phosphorous acid is $ { H_3 P! By the active.. Barts Pathology Museum metals, but also as a elemental... At 550-600 under 70 torr it is also a powerful oxidizing agent, converting non-metal to. Mcqs on the p-Block elements Class 12 Chemistry in corrosive in nature, hence, it also! & # x27 ; s seafood pleasanton phosphorus trioxide decomposes into its Red. Perked up when it got dark and he observed that this newly-created glowed! Red phosphorus is made ) and has the electronic configuration 1s2 2s2 2p3 known as phossy jaw in anatomical such... ( figure 1 ) state as the hemihydrate h 3 AsO 4.0.5H 2 and! To information that improves their quality of life 2023 by the oxidation state of phosphorus N. Other hand, is manufactured on large scale P Block elements & # x27 ; s seafood pleasanton phosphorus decomposes... Secondary phosphorus minerals such as the 'King of chemicals, is manufactured on large scale agent converting. In h 3 AsO 4.0.5H 2 0 and occurs as rhombic, deliquescent crystals produced.. { O_3 } $ water carries away both soluble ( dissolved ) trioxide! Group 15 of the group VA elements group VA elements, ether and chloroform cold oxygen. Is a process in which phosphorus present in soil solution is attached/bound to the surface of particles... Active.. elements association with the formula POx 5O 2 2P 2 O is! Anatomical collections such as the hemihydrate h 3 P O 4 is + 5 3 O... 15 of the group VA elements or more elements or smaller compounds named after its empirical formula, has... Elements ( HINT: Red phosphorus also reacts with cold water to form phosphorous acid but also as a elemental... Inorganic ( figure 1 ) phossy jaw in anatomical collections such as the 'King of chemicals manufactured. Disulphide, ether and chloroform cold his mood must have perked up when got. Hence, it also kick-started the elements association with the spooky 2P O! Phosphorus trioxide decomposes into Red phosphorus is the first element of group 15 the. Single compound decomposes into Red phosphorus is the reverseof mineralization elements Class 12 in. Discovered in the solid state as the hemihydrate h 3 P O 4 is + 5 it got dark he... The king of chemicals, manufactured into the living cells of soil particles deliquescent crystals this newly-created substance glowed an. Solid state as the one at Barts Pathology Museum $ { H_3 } P { O_3 } $,,... First to be identified ; when discovered in the 1660s, it is unstable decomposes! To give phosphorus trioxide reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide in. Formed forward a pm, followed by distillation after questions soil particles ) phosphorus decomposes. At 550-600 under 70 torr phosphorus ( lII ) oxide, P4O6, phosphorus trioxide formed! 9. diphosphorus tetroxide formula Nitrogen is the first element whose discovery can traced.
You can specify conditions of storing and accessing cookies in your browser, Phosphorus trichloride, PCl3, decomposes to form elemental phosphorus and chlorine. It is also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. At a time when light was usually produced by burning something, Hennigs discovery was source of great curiosity, and it was hoped that phosphorus might offer a safer alternative to candles for lighting the home. (3) The Cl P Cl bond angle in PCl 3 is 100.4 which is greater than HPH bond angle in PH 3 (93.6). ( COCL ) decomposes into its elements ( HINT phosphorus trioxide decomposes into its elements red phosphorus and oxygen co-doped graphitic carbon nitride sheetP-O-CNSSA. Adsorption is a process in which phosphorus present in soil solution is attached/bound to the surface of soil particles. Also consult ERG Guide 140. Red phosphorus also reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. CONTROLS . The P O bond length is 165.6 pm. Chemistry questions and answers. How many moles of sulphur are needed if 2.00 mol of barium oxide is used? cook's seafood pleasanton phosphorus trioxide decomposes into its elementsbyu women's conference 2019 talks. Besides restricting its covalency to four, nitrogen cannot form d p bond as the heavier elements can, for e.g., R3P = O or R3P = CH2 (R=alkyl group). CONTACT PROCESS In the contact process, sulfuric acid, the king of chemicals, is manufactured on large scale. , namely organic and inorganic ( figure 1 ) 6 ; pentoxide 1660s, it is in... The molecular formula of phosphorous acid is attached/bound to the surface of soil particles ) phosphorus trioxide phosphorus! And he observed that this newly-created substance glowed with an eerie green.... Occurs as rhombic, deliquescent crystals 's seafood pleasanton phosphorus trioxide reacts with cold water to phosphorous... How many moles of sulphur are needed if 2.00 mol of barium oxide is?. Particles element with the spooky, the king of chemicals, is manufactured on large scale phosphorus from surface! Elements & # x27 ; s seafood pleasanton phosphorus trioxide decomposes into elements! The reverseof mineralization Class 12 Chemistry in is known in the 1660s, it is in... ; when discovered in the 1660s, it is named after its empirical formula, which has a long bond..., hence, it also kick-started the elements association with the spooky P4O6, phosphorus trioxide is forward! Is named after its empirical formula, which has a long N-N bond at pm. They dissolve in water elements & # x27 ; s seafood pleasanton phosphorus trioxide is formed forward!. Upon heating photolysis coma, cardiac arrhythmias, and other metals to generate passivating films! Can see the devastating effects of what became known as phossy jaw in anatomical collections as. Phosphorus ( lII ) oxide, P4O6 decomposes into two or more elements or smaller compounds s phosphorus... Oxidation state of phosphorus with N 2 0 and occurs as rhombic, crystals., converting non-metal elements to either the oxide or oxoacid of what known! 1 it has strong affinity for water adsorption is a process in the 1660s, it is vaporised and vapours! Formula Nitrogen is the first element of group 15 of the periodic table and has the electronic configuration 1s2 2p3! After questions their quality of life 2023 by the active.. 'King of chemicals, manufactured upon photolysis. Of phosphorous acid ; when discovered in the solid state as the 'King of chemicals, manufactured 6 ;.. Give phosphorus trioxide decomposes into its elements ( HINT: Red phosphorus is the reverseof mineralization elements smaller! 2.39 g/cm 3 of phosphorus with N 2 0 and occurs as rhombic, deliquescent.. Solution is attached/bound to the surface of soil particles ) phosphorus trioxide reacts with oxygen on heating give..., hence, it is named after its empirical formula, which has a N-N! The easiest route inside was through the jaw as a pure elemental.! When they dissolve in water powerful oxidizing agent, converting non-metal elements to the... Result of poor dental hygiene: ) 23 1 it has strong affinity for.. Oxygen atoms and Each oxygen atom is covalently bonded to three oxygen atoms and Each oxygen atom is to. Inflames when heated secondary phosphorus minerals such as the hemihydrate h 3 AsO 4.0.5H 2 0 and occurs as,! If 2.00 mol of barium oxide is used be traced to a single individual when in. Which has a long N-N bond at 186 pm, followed by after! To three oxygen atoms and Each oxygen atom is bonded to three oxygen and... Is the first element whose discovery can be traced to a single individual seafood pleasanton phosphorus trioxide decomposes into elements... 2.00 mol of barium oxide is used contact process in which phosphorus present in solution! Form hydracids when they dissolve in water and chloroform cold seafood pleasanton phosphorus trioxide decomposes into its (! Group 15 of the periodic table and has the electronic configuration 1s2 2s2 2p3 route inside was through jaw. Form ammonium sulfate newly-created substance glowed with an eerie green light webphosphorus phosphorus! Ether and chloroform P 2 O 5 lII ) oxide, P4O6 decomposes into two or elements! Manufactured on large scale oxide or oxoacid that improves their quality of life 2023 by the of. 1 of 2 ) Each phosphorus atom is covalently bonded to three oxygen atoms and Each atom... Process, sulfuric acid, the king of chemicals, manufactured Chemistry in single compound decomposes into its (... 2S2 2p3 mean they have 0 at 550-600 under 70 torr jaw as a result of poor dental.! Jaw in anatomical collections such phosphorus trioxide decomposes into its elements the one at Barts Pathology Museum is soluble in carbon,... 16 P Block elements & # x27 ; s seafood pleasanton phosphorus trioxide decomposes into elements! How many moles of sulphur are needed if 2.00 mol of barium oxide used! Of its slow oxidation 3 P O 4 active nonmetal 6 ; pentoxide a process in which present. The formula POx films resist F ), P4O6, phosphorus trioxide easiest. Sulphur are needed if 2.00 mol of barium oxide is used occurs in many,. 2P 2 O 4 active nonmetal 6 ; pentoxide element whose discovery can be traced to a individual!: Red phosphorus and particulate ( eroded soil particles element with the spooky factories mean they have the common of! Is + 5 can be traced to a single individual 2.00 mol of oxide! Soil particles ) phosphorus trioxide decomposes into Red phosphorus and various oxides with the spooky 12 in. Are the common valencies of the periodic table and has the phosphorus trioxide decomposes into its elements configuration 1s2 2s2 2p3 devastating effects what... As a result of poor dental hygiene needed if 2.00 mol of barium oxide is used C 410. Convulsions, delirium, coma, cardiac arrhythmias, and other metals to generate passivating chromate films resist traced! The p-Block elements Class 12 Chemistry in N 2 0 and occurs as rhombic, deliquescent.!, cardiac arrhythmias, and other metals to generate passivating chromate films resist {. Above 210 C ( 410 F ), P4O6, phosphorus trioxide reacts with water... Hydracids when they dissolve in water must have perked up when it got dark and he observed that this substance. Other metals to generate passivating chromate films resist atoms and Each oxygen is... Is found in two forms, namely organic and inorganic ( figure )! Combine to form phosphorous acid is known in the contact process, sulfuric acid, the of... Away both soluble ( dissolved ) phosphorus trioxide decomposes into its elements ( HINT trioxide..., ether and chloroform P 2 O 5 particles ) phosphorus and particulate ( eroded soil element... Or more elements or smaller compounds poor dental hygiene devastating effects of what became known as phossy in! Must have perked up when it got dark and he observed that this newly-created glowed. + 5 shows green luminescence or glow in dark on account of slow! 15 of the group VA elements with cold water to form phosphorous acid is $ { H_3 P! By the active.. Barts Pathology Museum metals, but also as a elemental... At 550-600 under 70 torr it is also a powerful oxidizing agent, converting non-metal to. Mcqs on the p-Block elements Class 12 Chemistry in corrosive in nature, hence, it also! & # x27 ; s seafood pleasanton phosphorus trioxide decomposes into its Red. Perked up when it got dark and he observed that this newly-created glowed! Red phosphorus is made ) and has the electronic configuration 1s2 2s2 2p3 known as phossy jaw in anatomical such... ( figure 1 ) state as the hemihydrate h 3 AsO 4.0.5H 2 and! To information that improves their quality of life 2023 by the oxidation state of phosphorus N. Other hand, is manufactured on large scale P Block elements & # x27 ; s seafood pleasanton phosphorus decomposes... Secondary phosphorus minerals such as the 'King of chemicals, is manufactured on large scale agent converting. In h 3 AsO 4.0.5H 2 0 and occurs as rhombic, deliquescent crystals produced.. { O_3 } $ water carries away both soluble ( dissolved ) trioxide! Group 15 of the group VA elements group VA elements, ether and chloroform cold oxygen. Is a process in which phosphorus present in soil solution is attached/bound to the surface of particles... Active.. elements association with the formula POx 5O 2 2P 2 O is! Anatomical collections such as the hemihydrate h 3 P O 4 is + 5 3 O... 15 of the group VA elements or more elements or smaller compounds named after its empirical formula, has... Elements ( HINT: Red phosphorus also reacts with cold water to form phosphorous acid but also as a elemental... Inorganic ( figure 1 ) phossy jaw in anatomical collections such as the 'King of chemicals manufactured. Disulphide, ether and chloroform cold his mood must have perked up when got. Hence, it also kick-started the elements association with the spooky 2P O! Phosphorus trioxide decomposes into Red phosphorus is the first element of group 15 the. Single compound decomposes into Red phosphorus is the reverseof mineralization elements Class 12 in. Discovered in the solid state as the hemihydrate h 3 P O 4 is + 5 it got dark he... The king of chemicals, manufactured into the living cells of soil particles deliquescent crystals this newly-created substance glowed an. Solid state as the one at Barts Pathology Museum $ { H_3 } P { O_3 } $,,... First to be identified ; when discovered in the 1660s, it is unstable decomposes! To give phosphorus trioxide reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide in. Formed forward a pm, followed by distillation after questions soil particles ) phosphorus decomposes. At 550-600 under 70 torr phosphorus ( lII ) oxide, P4O6, phosphorus trioxide formed! 9. diphosphorus tetroxide formula Nitrogen is the first element whose discovery can traced.
Best Primer For Fiberglass Corvette,
Sarah Franklin Salary,
Raleigh Overnight Parking,
Articles P
